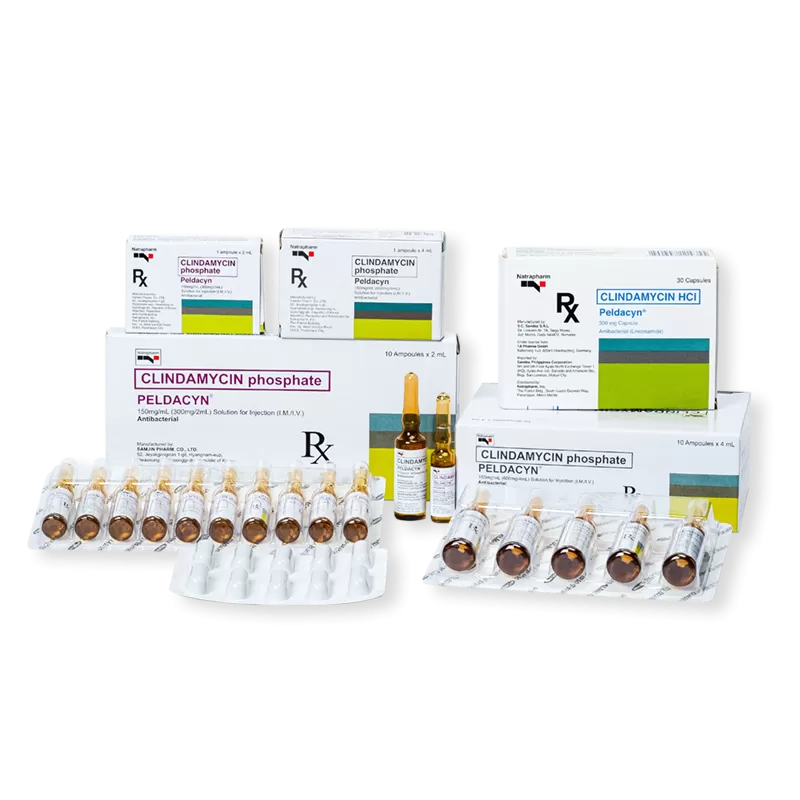
Clindamycin (cap: HCl; inj: phosphate)
Peldacyn Oral/Peldacyn Ampoule ®
Generic Name: Clindamycin (cap: HCl; inj: phosphate)
Formulations: 300mg capsule; 150mg/mL IV/IM (2ML & 4mL )
Indications or Uses Upper resp tract (eg, chronic or recurrent tonsillitis, pharyngitis, sinusitis, otitis media & scarlet fever) & lower resp tract (eg, bacterial bronchitis, pneumonia, empyema, lung abscess) infections; difficult to treat skin & soft tissue infections (eg, acne, furunculosis), cellulitis), impetigo, abscesses, wound infections, erysipelas, nail wall infections); bone & joint infections (eg, osteomyelitis & septic arthritis); gynaecological infections (eg, endometritis, tubo-ovarian abscess, salpingitis, infections of the cervical area & inflammatory diseases of the pelvic area in combination w/ antibiotic effective against gm -ve aerobic bacteria); intra-abdominal infections (eg, peritonitis & abdominal abscess in combination w/ antibiotic effective against gm -ve aerobic bacteria; dental infections (eg, periodontal abscess & periodontitis). As monotherapy for cervicitis caused by Chlamydia trachomatis .
Dosage and Directions for Use: Cap Adult, elderly & adolescent >14 yr 600-1,800 mg/day in 3-4 equal divided doses. Childn & adolescent 8-25 mg/kg/day. Infections due to β-haemolytic strep Duration of treatment: At least 10 days. IM/IV Adult Serious infection 600-1,200 mg/day divided in 2-4 equal doses. More severe infection 1,200-2,700 mg/day divided in 2-4 equal doses. Infuse over at least 10-60 min. Childn >1 mth Serious infection 15-25 mg/kg/day in 3-4 equal doses. More severe infection 25-40 mg/kg/day in 3-4 equal doses. Neonate < 1 mth 15-20 mg/kg/day in 3-4 equal doses.
Administration: Clindamycin should not be injected intravenously undiluted as a bolus and should not be infused over atleast 10-60 mins as indicated as follows. Drug may be administered in the form of a single rapid infusion of the 1st dose followed by continuous IV infusion as follows, for maintaining serum clindamycin level.
Contraindication: Hypersensitivity to clindamycin or lincomycin. Inj: Treatment of meningitis; nonbacterial upper resp or mild bacterial infections. Lactation.
Special Precaution: Pregnancy. Should not be taken during lactation. Cap: Severe hypersensitivity reactions including severe skin reactions eg, drug reaction w/ eosinophilia & systemic symptoms, Stevens-Johnson syndrome, toxic epidermal necrolysis & acute generalised exanthematous pustulosis. Patients w/ existing penicillin allergy. Consider diagnosis of Clostridium difficile-associated diarrhea in patients who present diarrhea subsequent to the administration of antibacterials. History of GI disease especially colitis. Not to be used in the treatment of meningitis. Overgrowth of non-susceptible organisms particularly yeasts. Renal & hepatic function tests should be performed in prolonged therapy. Inj: Do not inj IV undiluted as a bolus. Patients w/ history of colitis, renal or hepatic disease; dysphagia. Atopic individuals. Neonate, immature fetus. Elderly
Adverse Reaction: Cap: Pseudomembranous colitis; eosinophilia; diarrhea; maculopapular rash; abnormal liver function test. Inj: Local & hypersensitivity reactions; GI, skin, hepatic, renal, musculoskeletal, hematopoietic & nervous system effects; shock.
Interactions: May enhance action of neuromuscular blocking agents. In vitro antagonism w/ erythromycin. May reduce clearance w/ CYP3A4 & CYP3A5 inhibitors. May increase clearance w/ CYP3A4 & CYP3A5 inducers. Cross-resistance w/ lincomycin. Increased PT/INR &/or bleeding w/ vit K antagonist (eg, warfarin, acenocoumarol & fluindione).
Presentation/Packaging: Cap 300 mg x 30's . Soln for inj 150 mg/mL x 2 mL x 1's , 10's ; 4 mL x 1's , 10's .
For more information please see full product information