|
Home » Products » Fixcom 4
IMPORTANT: THIS IS A PRESCRIPTION DRUG.
Caution: Foods, Drugs, Devices and Cosmetics Act prohibit dispensing without prescription.
The contents of this page are provided for information purposes only. It should not be construed as a substitute for a professional medical advice. Please consult your physician and other healthcare providers before taking any medicines found in this page.
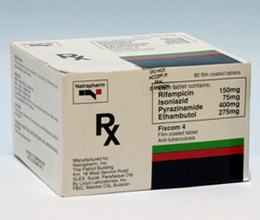
Fixcom 4®
Film-coated tablet
|

